cGMP/Personnel
PERSONNEL
Person responsible for supervising the manufacture, processing, packing, or holding of a drug product shall have the education, training, and experience, or any combination thereof, to perform assigned functions in such a manner as to provide assurance that the drug product has the safety, identity, strength, quality, and purity that it represents to possess.
EDUCATION
QUALIFIED STAFF NUMBERS
EXPERIENCE
RESPOSIBILITIES
TRAINING
CONSULTANTS
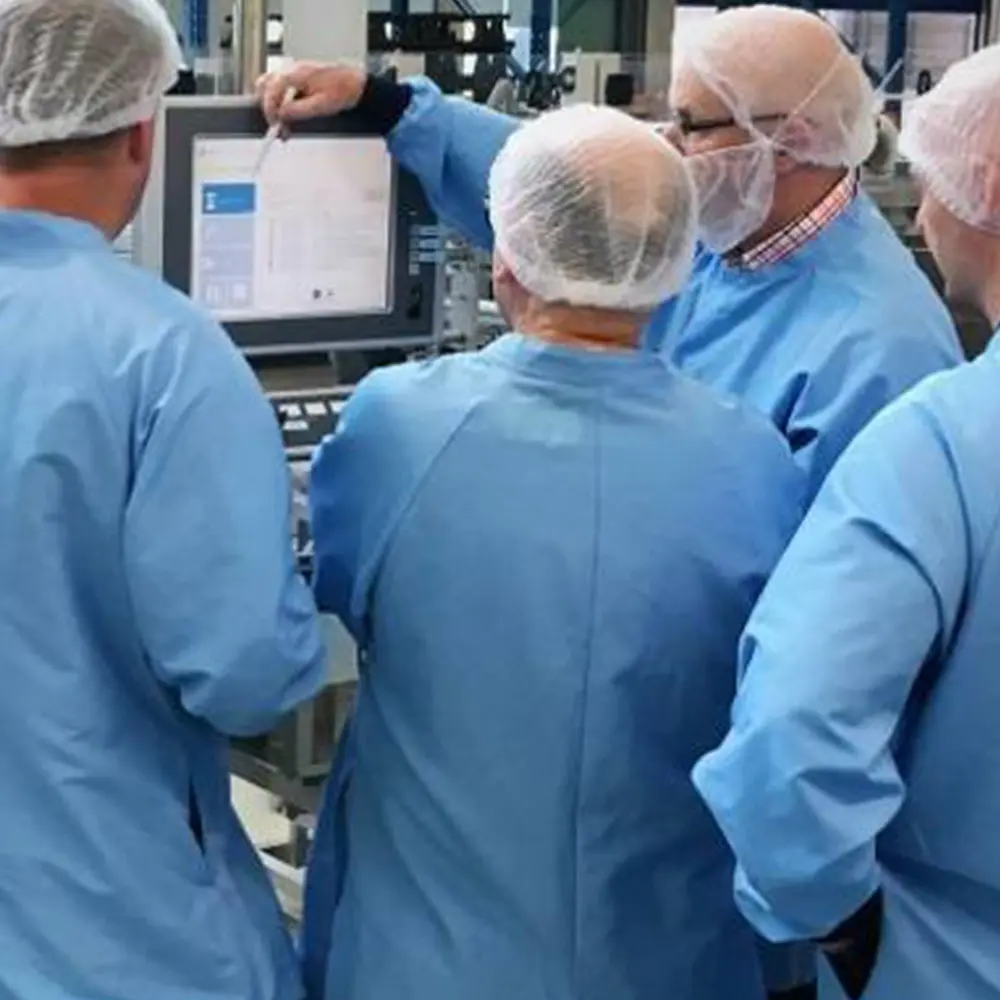
EDUCATION i EXPERIENCE i TRAINING
Each person engaged in the manufacture, processing, packing, or holding of a drug product shall have education, training, and experience, or any combination thereof, to enable that person to perform the assigned functions.
- Training on
- cGMP requirements
- Written Procedures as they relate to employee
- Training By
- Qualified Trainer
- Training Frequency
- Sufficient to keep employees familiar with cGMP and written procedures

RESPONSIBILITIES
- Good sanitation practices like Shower, Hand wash, Hair & Nails cut etc.)
- Clean clothing appropriate to perform duties
- Protective apparel (Head covers masks googles etc. to protect drug products from contamination
- Limited accessibility to designated areas only
- Regular Medical checkups
- Personnel with disease condition or open lesions shall be excluded from direct contact with starting or inprocess materials and drug products.
Phone
03334115785
Location
554, Sundar Industrial Estate Lahore
info@pmdconsultancy.com
